CONTENT MIGRATION FOR STRUCTURED COMPONENT AUTHORING
STRUCTURED COMPONENT AUTHORING

OUR SOLUTION ACCELERATED OUR CUSTOMER TO ACHIEVE OVER 80% CONTENT REUSE
THE PROBLEM
Structured component authoring (SCA) is the most efficient way to write, review, and publish documents and content. SCA has not been adopted well within Life Sciences. This has been largely due to:
- Authoring interfaces behave more like software developer tools rather than document authoring tools and
- Authoring documents in a structured format to generate the proposed content reuse return on investment may take years
The Docuvera platform has made major progress in producing a highly intuitive and adoptable user interface that addresses the main barrier to SCA adoption. However, the problem of the long lag time to achieve the ROI still exists when starting with an empty library. Most legacy documents live in Microsoft Word; despite efforts to maintain standards around structure, Word offers writers too much freedom to not adhere to well-intentioned standards rules. This makes migrating the existing Word content into a form suitable for SCA extremely daunting.
OUR SOLUTION
Entitech Solutions delivered the bridge to easily move authors from Word to structured authoring.
Solution includes:
- Conversion of Word documents into reusable components via a robust authoring and content conversion rules engine.
- Tabular view of a document with all its components broken out, once converted, e.g., text, characteristics, and hierarchies, simplifying steps to apply content componentization rules.
- Content import utility to bring rules into a structured authoring tool.
- Component de-duplication and existing reuse detection.
THE RESULTS
- Time Savings: Extensive time savings via automation of componentizing the content in thousands of documents via the solution rules engine.
- Productivity gains: Improvements in writing new content using components.
- Reuse simplified: Immediate access to multiple years’ worth of content components ready for reuse by writers and content administrators in new documents.
- Speed: Ability to load and componentize content from over 5,000 pharmaceutical scientific response documents within a few months – vs. potential of years without the solution.
- This solution further deployed for other customers, and across other domains such as clinical trial documentation, labeling, and safety reporting.

Pharmaceutical Scientific Response Documents Converted for reuse and immediately available day one
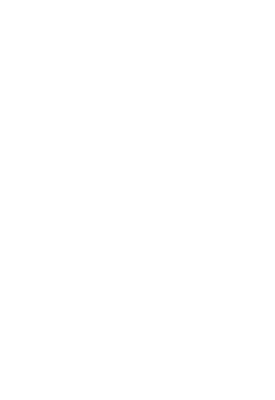
Reduction in manual effort during migration
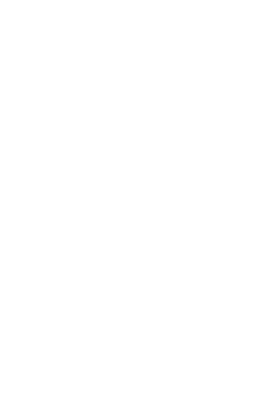
Content Reuse Achieved


