Ready for Reuse — Questions to Ask Before Adopting a Structured Component Authoring Platform
Ask yourself the following questions about content reuse before you dive into structured component authoring.

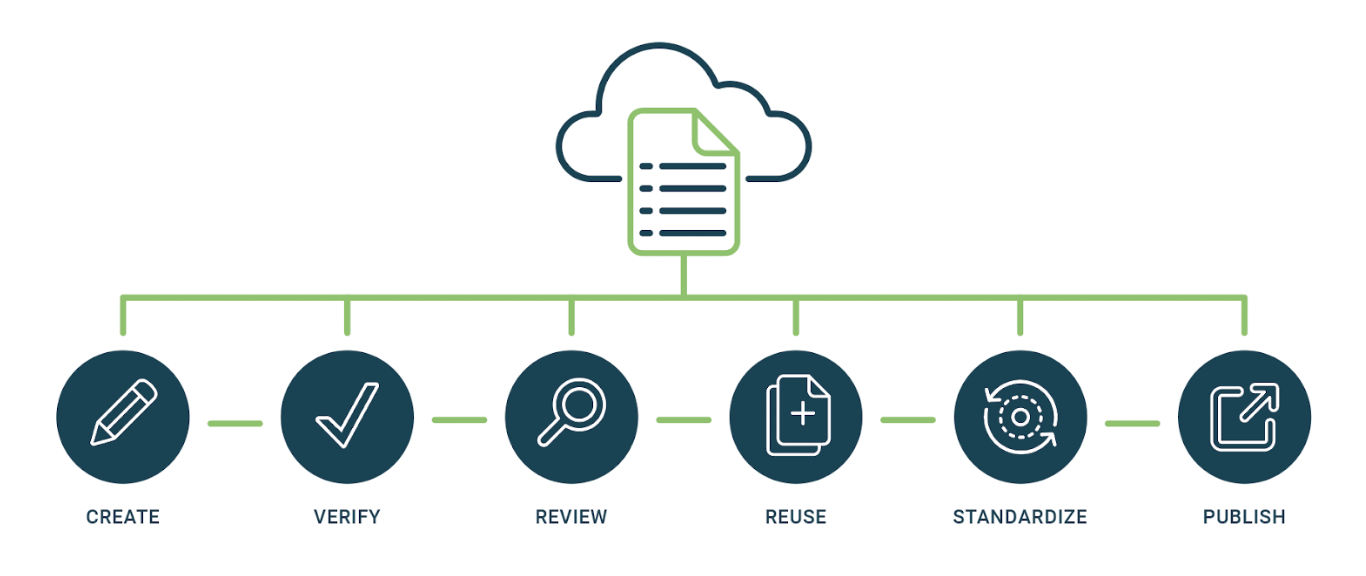
Structured component authoring can be transformative for Life Science writers who create clinical documents, compose files related to medical information, or work with any other content that requires extensive documentation and regulatory review.
In Structured Component Authoring 101, we outlined many key definitions and benefits, including:
- Easily verifying content
- Simplifying regulatory reviews
- Quickly finding and organizing content components
- Maintaining consistency in styles and formats
An additional and important benefit to consider is content reuse. Content reuse is the key to consistency and compliance across documents in a life science organization.
These features sound appealing, but you may still find yourself wondering whether structured component authoring is right for your situation. To find the answer, ask yourself the following questions:
Do you reuse content often?
If your Life Science company reuses content often, adopting a structured component authoring solution is a no-brainer.
Many authors write dozens of documents about a given product, from regulatory documents, to protocols, and submissions. As information changes, authors need to keep up with the various changes and continuously do cross-checks to ensure that information matches across all related documents.
The right structured component authoring solution will enable content authors to reuse the same content error-free by using component libraries to verify and organize content to copy. This is not a copy/paste function, but rather a metadata capability that drives consistency and contemporaneous content.
When properly implemented, structured component authoring can help these authors improve efficiency and accuracy of this information. Structured component authoring lets users update a given component and propagate that change everywhere, in every document where that component is used.
Do you know how your content is being reused by others?
Your documentation or information may be used outside the purview of you or your Life Science company.
If this is the case, structured component authoring can help you reduce errors, minimize style format inconsistencies, and make it easy for collaborators to see the changes you made or make modifications based on source component changes.
Is your company willing to reuse content in different ways?
Some Life Science companies take content beyond creating and accessing documents. They also serve audiences on channels including websites, chat bots, call centers, and infographics. If you are trying to provide a true omnichannel experience to your end users, structured authoring really lets you provide a consistent message across multiple channels.
In order to have an effective omnichannel strategy like this, Life Science companies need to change the way content is created and managed through the development life cycle. Structured component authoring makes it easy for users to separate components and metadata from each presentation, so all content can be effectively edited and delivered across all channels.
Entitech can help
Entitech Solutions (ETS) can make these reuse capabilities a part of your organization’s content management through structured component authoring.
We have been involved in several implementations, and our solution has helped clients achieve over 80% content reuse. Learn how we can help you. Request a consultation today.
